Bioneer's siRNA Drug Development Program using SAMiRNA Technology
Vertically integrated processes within Bioneer's siRNA Drug Development Program provide a total solution for siRNA therapeutics discovery and development,
from siRNA design/synthesis and preclinical tests to IND filing.
With its world's-best RNAi core technologies and manufacturing infrastructure, Bioneer is the ideal partner for pharmaceutical and biotechnology
companies currently working on the development of RNAi therapeutics, or seeking to enter the RNAi field. Bioneer's research and technical
support teams ensure top-quality products and services to meet your unique needs.
Our siRNA Drug Development Program includes:
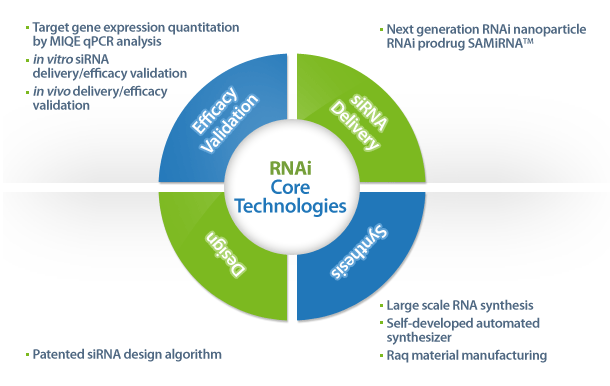
- Design and synthesis of siRNAs (custom siRNA synthesis using patented design algorithm, which has been used for
genome-wide siRNA libraries for human, mouse and rat)
- in vitro screening and validation of siRNAs including MIQE qPCR analysis of knockdown rates
- Preclinical test using SAMiRNA including in vivo siRNA delivery and biodistribution/efficacy test using murine and
non-human primate models
- Large scale manufacturing of SAMiRNA (in house raw material manufacturing facilities, fully automated siRNA/SAMiRNA
synthesis pipeline)
- Drug candidates efficacy/toxicity tests in nonhuman primate (through collaboration with Korea Institute of Toxicology)

Please click each step for more information