SAMiRNA™ (Self-Assembled-Micelle-inhibitory-RNA)
One of the major obstacles in the development of RNA interference (RNAi)-based therapeutics is siRNA/miRNA delivery.
SAMiRNA (Self-Assembled-Micelle-inhibitory-RNA) is a novel class of RNAi molecule developed by Bioneer,
which provides the solution for virtually all major difficulties in the development of RNAi-based therapeutics including siRNA delivery.
SAMiRNA is prodrug that is manufactured as a single chemical entity and spontaneously Self-Assembles into nanoparticles with a protective PEG coat.
SAMiRNA nanoparticles can incorporate siRNA, miRNA or even antisense DNA, and are the ideal size for selective localization in either vascularized tumors,
(via Enhanced Permeability and Retention (EPR) effects),
or to targeted tissues through the addition of targeting moieties to the surface of SAMiRNA.
The therapeutic potential of SAMiRNA is outstanding because of its negligible toxicity and outstanding in vivo serum stability as well as its efficacy
in target gene silencing. Our data demonstrate SAMiRNA's exceptional therapeutic potential as an RNAi platform for a variety of diseases.
Bioneer is uniquely positioned for the development and advancement of our SAMiRNA system as it combines our extensive pre-clinical research
expertise with our nucleic acid manufacturing and development expertise:
Bioneer has been providing top-quality DNA oligos and siRNAs worldwide for 20 years.
Using this novel RNAi prodrug technology, Bioneer is currently advancing clinical development of pipeline programs for previously non-druggable
diseases through our own pipeline efforts as well as through partnerships with major global pharmaceutical companies.
Key features of SAMiRNA™
: Best-in-class RNAi drug SAMiRNA overcomes the Unmet needs in siRNA drug development
| Adverse effects |
No detectable liver toxicity or innate immune response |
| Delivery (Issues with other technologies: rapid clearance and degradation in blood,
No target tissue specificity, low in vivo efficacy) |
Outstanding serum stability (PK/PD validated),
tumor/tissue targeting capabilities verified,
long lasting in vivo efficacy validated in animal disease models |
| Manufacturing cost |
One-step automated solid-phase synthesis of SAMiRNA and rapid chromatographic purification process:
enormous advantage allowing for economical large scale production of SAMiRNA. |
| QC processes |
Since SAMiRNA is a Single Chemical Entity the QC process is dramatically simplified |
| Expansion of indications |
Powerful siRNA delivery platform technology with flexibility to incorporate siRNA (or miRNA or antisense)
sequences against any disease target; Your target gene(s) can be easily transformed into SAMiRNA nanoparticle! |
SAMiRNA™ Summary
Best-in-class RNAi prodrug technology: SAMiRNA™ is the most unique and effective RNAi prodrug developed to date for the treatment of cancer and other diseases.
Pre-clinical data for SAMiRNA
 Novel self-Assembling siRNA nanoparticles with a protective PEG coat and optimal monodisperse nano-scale size
Novel self-Assembling siRNA nanoparticles with a protective PEG coat and optimal monodisperse nano-scale size
(Figure 1)
 siRNA Prodrug: highly stable in circulation with siRNA release and activity only after metabolism within target cells
siRNA Prodrug: highly stable in circulation with siRNA release and activity only after metabolism within target cells
 Outstanding serum stability (Figure 4)
Outstanding serum stability (Figure 4)
 in vivo efficacy validated in animal disease models: completion of preclinical tests for cancer treatment (Figure 2)
in vivo efficacy validated in animal disease models: completion of preclinical tests for cancer treatment (Figure 2)
 Extremely low toxicity and cytokine induction (Figure 5-7)
Extremely low toxicity and cytokine induction (Figure 5-7)
 Combined with various targeting moieties, SAMiRNA nanoparticles can target various organs of interest, for example, liver
Combined with various targeting moieties, SAMiRNA nanoparticles can target various organs of interest, for example, liver
A new class of single molecule-based self-assembling Nanoparticles
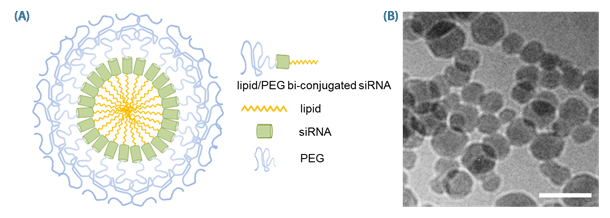
Figure 1. Structure of SAMiRNA Nanoparticle.
(A) Schematic diagram of SAMiRNA (B) Cryo-TEM images of SAM-siRNA Nanoparticles (scale bar = 100 nm).
Long-lasting in vivo efficacy validated in mouse cancer models
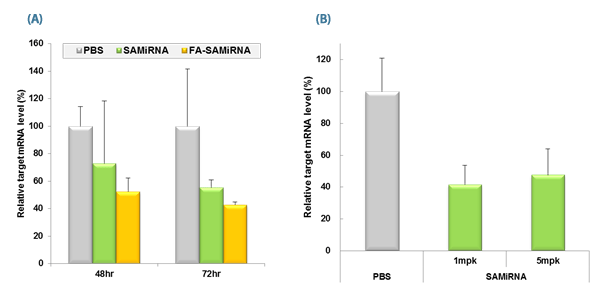
Figure 2. in vivo silencing of target mRNA by SAMiRNA Nanoparticels.
(A) Tumor bearing mice were intravenously injected with saline (PBS), or with a single 5mg/kg dose of either survivin-SAMiRNA, or tumor-targeting folic acid (FA)-conjugated survivin-SAMiRNA.
Mice were sacrificed at denoted time points and survivin mRNA levels in isolated tumor cell masses were subsequently measured using Real-Time PCR.
(B) Tumor bearing mice were intravenously injected with saline (PBS) and survivin-SAMiRNA NP at a single 1 or 5mg/kg dose.
Mice were sacrificed at 72 hr postinjection and survivin mRNA levels in isolated tumor cell mass were subsequently measured using real-Time PCR.
Tumor-specific targeting of SAMiRNA in mouse cancer models
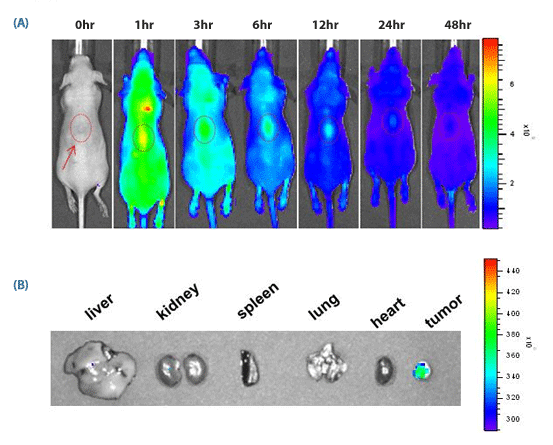
Figure 3. in vivo targeting of SAMiRNA Nanoparticles to s.c. grafted tumor.
(A) Time-dependent in vivo tumor targeting specificity of Cyanine3.5-labeled SAMiRNA, delivered via i.v. to tumor-bearing nude mice.
(B) Images of various organs extracted from treated mice 12 hours after injection. No fluorescence was detectable outside of the tumor proper.
in vivo PK/PD Quantification of i.v. administrated SAMiRNA™
Shows significantly enhanced stability compared to modified siRNA (2'-O-Methyl siRNA)
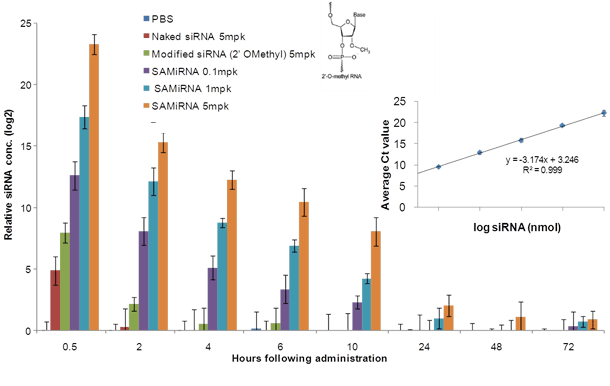
Figure 4. Quantification of siRNA in plasma from mice i.v. injected with SAMiRNA.
Mouse blood was extracted at indicated time points (hours post-injection) and SAMiRNA and siRNA levels in isolated plasma were measured by qRT-PCR assay.
The linear regression of the amplification curve shows an excellent R2 value for this assay.
SAMiRNA™ has no detectable adverse effects
Did not trigger any IFN response or liver toxicity
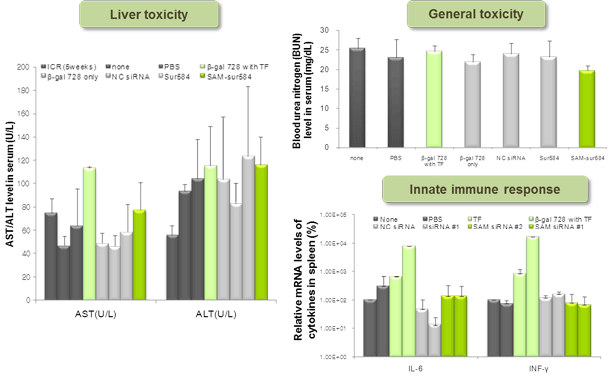
Figure 5. General toxicity test of SAMiRNA
Naked siRNA and SAMiRNA were i.v. injected into ICR mice in order to evaluate toxicity. Serum was collected 6 or 24 hr post-injection and toxicity markers were analyzed.
None (not-injected), ICR 5 weeks (average value from normal 5-week old ICR mice, from published literature), b-gal 728 (beta-galactosidase siRNA),
b-gal 728 with TF (beta-galactosidase siRNA mixed with in vivo MegaFectine from QBiogene), NC siRNA (negative control siRNA), Sur584 (survivin siRNA), SAM-sur584 (survivin siRNA-containing SAMiRNA).
SAMiRNA™ has no detectable adverse effects
Negligible cytokine induction in human PBMCs by SAMiRNA
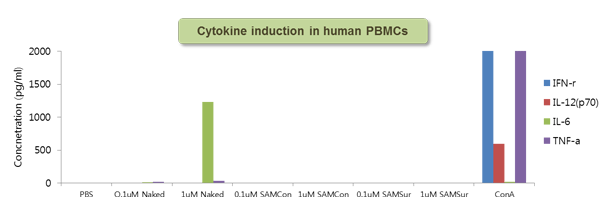
Figure 6. Cytokine response to SAMiRNA in human PBMC
Human whole blood was incubated with 0.1 or 1 uM of naked siRNA or SAMiRNA for 24 hrs at 37°C and the resulting supernatant was measured for various cytokine induction.
Concanavalin A (ConA) was used as positive control.
SAMiRNA™ has no detectable adverse effects
Extremely low toxicity profile of SAMiRNA
Independent results from a GLP-certified CRO, Biotoxtech Co. Ltd)
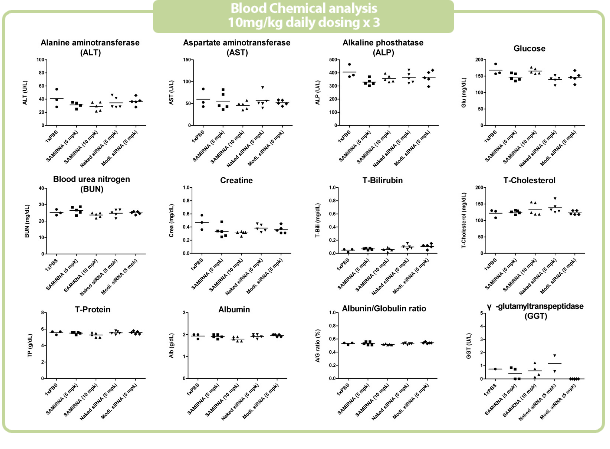
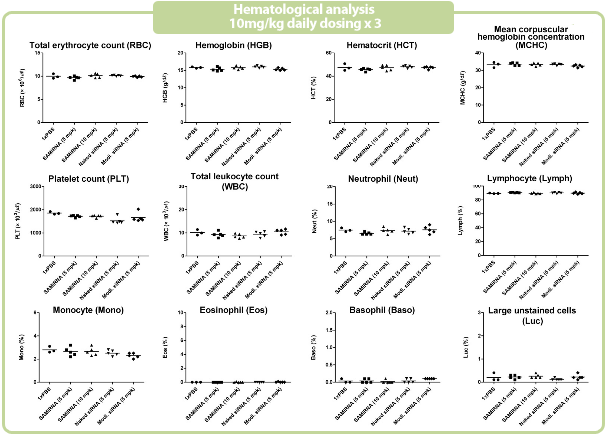
Figure 7. in vivo SAMiRNA blood chemical and hematological analysis
10 mpk of SAMiRNA, naked or modified siRNA was repeatedly i.v. injected to ICR mice (n=5) 3 times at intervals of one day.
Whole blood and separated serum were collected and analyzed 24 hrs after the final injection.