The yeast Saccharomyces cerevisiae (S. cerevisiae) is a recognized simple eukaryote model system which genome can be easily manipulated.
The S. cerevisiae VN-Fusion Library was created by Dr. Won-Ki Huh of Seoul National University (Korea).
The VN-Fusion Library consists of 5,809 VN-tagged Open Reading Frames (ORFs) covering 93% of the yeast proteome.
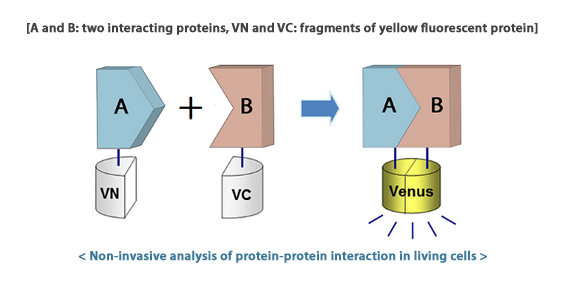
Bimolecular Fluorescence Complementation (BiFC) assay
Most biological processes are carried out and regulated by dynamic networks of protein-protein interactions.
The Bimolecular Fluorescence Complementation (BiFC) assay is now regarded as one of the most advanced and powerful tools for studying
in vivo detection of protein–protein interactions in several orgarnisms. The BiFC assay is based on the formation of a fluorescent complex
by fragments of yellow fluorescent protein, brought together by association of two interacting partners fused to the fragments.
This approach enables visualization of the subcellular localizations of specific protein complexes in the normal intracellular environment.
Features and Benefits
A powerful tool for studying protein-protein interactions in living cells
- Non-invasive method for analyzing fluorescence without the need for any external cofactors
Clear visualization of subcellular protein-protein interaction localization
- Formation of a fluorescent complex
Stronger signal and direct readout measurable with relatively simple equipment
- Using a fluorescence microscope
Genome-wide high-throughput screening possible
- 93% yeast proteome coverage
Unknown protein function analysis through functional complementation is possible
Analysis of proteinylation (ubiquitination, sumoylation, neddylation) is possible